Unit 1: Atomic Structure
Energy is Quantized!
- Formula: E = hf = hc/λ
- Wavelength and energy relationships:
- UV > Vl > IR (in terms of energy and frequency)
- Wavelength increases in the order: UV < Vl < IR
Photoelectric Effect
- Light photons of specific wavelengths or frequencies are used to excite or eject electrons.
- Formula: Energy of photons = B.E (Binding Energy) + K.E (Kinetic Energy).
Coulombic Force
- Coulomb’s Law: q₁q₂ / r
- Ionic, metallic, Lattice, Hydration
Electron Configuration
- 1s 2s 2p 3s 3p 4s 3d
- Cr , Cu exception
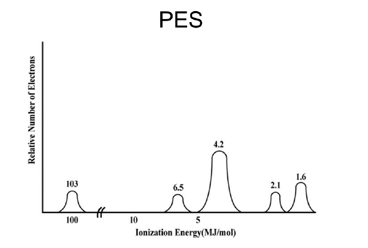
Photoelectron Spectroscopy (PES)
- Intensity (y-axis) corresponds to the number of electrons.
- Binding energy (x-axis) represents electron removal energy.
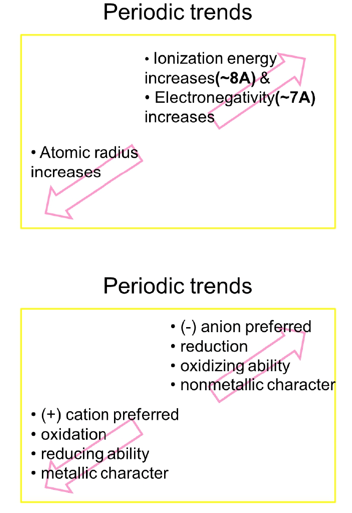
Periodic Trends
Proton no = Nuclear charge
Shell no = Energy level = Shielding no
Ions – HO¹⁻, NH₄¹⁺, NO₃¹⁻ , CO₃²⁻ , SO₄²⁻ , PO₄³⁻
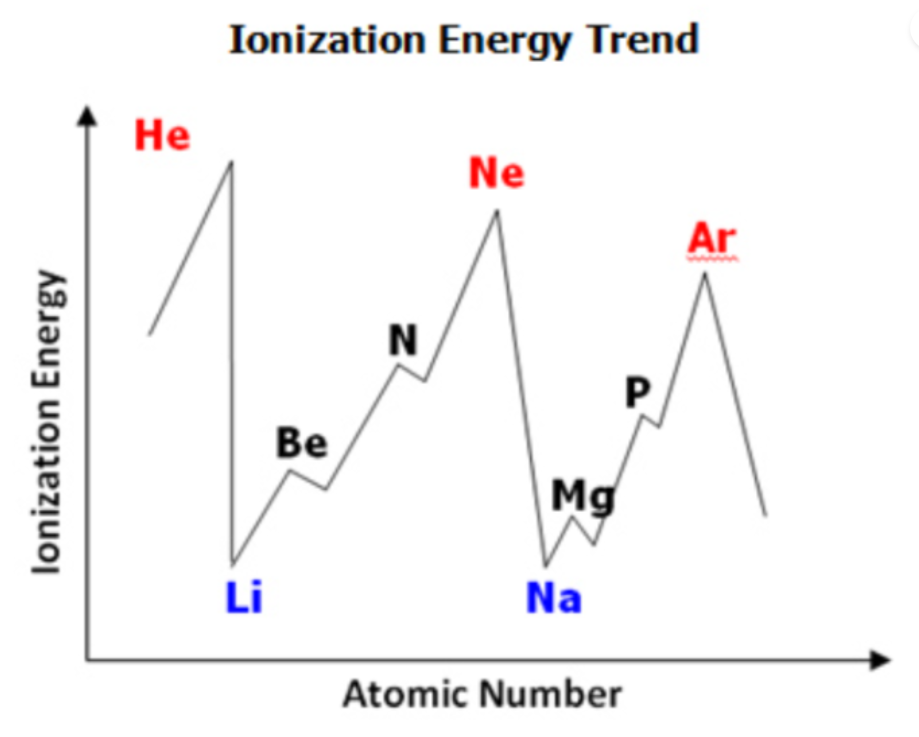
Ionization energy Excemption
- Ionization energy values decrease:
- From Be to B (due to p orbital initiation).
- From N to O (due to paired electrons in p orbital).
- Electron Affinity ( Exothermic)
Mass Spectrometer
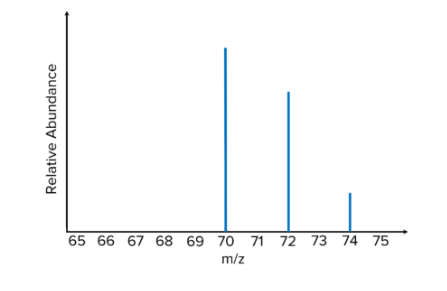
- Calculates average atomic mass.
- Example: Bromine isotopes
- ⁷⁹Br (50.5%)
- ⁸¹Br (49.5%)
Unit 2: Chemical Bonding
Metallic Bond
- Free electrons = delocalized electrons = valence electrons.
- Examples: Li < Mg (bond strength – Coloumb law)
- Metal related to Ionization Energy , Nonmetal related to Electron Affinity
- Malleability and Ductility
- Types:
- Interstitial Alloy: Different radii, more rigid.
- Substitutional Alloy: Comparable radii, malleable, ductile.
Ionic Bond
- Transferring electron(s)
- Bond strength proportional to Coulomb’s law.
- Examples: LiF > NaCl , LiF < MgO.
- Lattice Energy:
- Formula: XY(s) → X⁺(g) + Y⁻(g).
- lattice stucture
Covalent Bond
- Sharing electrons.
- Types:
- Network Covalent Solids: Ex) C (diamond, graphite), SiO₂ , Si
- Molecular Substances: Ex) H₂O, CO₂, N₂.
- allotrope vs isotope vs isomer
- Strength – Intermolecular Forces
| Solid Type | Metallic Solids | Ionic Solids | Molecular Solids |
|---|---|---|---|
| Properties | – Good conductors | – Do not conduct, but conduct when melted/ dissolved in water | – Do not conduct – Acid , Base and graphite exception |
| Malleable/ductile | Low vapor pressure | – Low melting point (Except to Giant covalent) | |
| – Brittle | – Composed of discrete molecules | ||
| Group 1, NO₃⁻ , NH₄⁺ Absolutely Dissolve | – “Like dissolves like” principle applies |
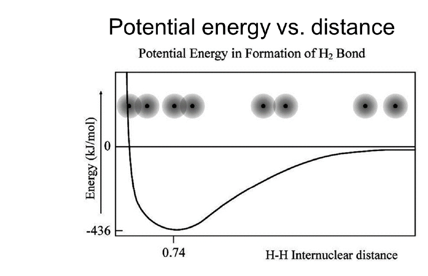
Covalent Bonds and Potential Energy
Lewis Struture
CH₄, CH₂=CH₂, NH₃ , NH₄⁺ , CH₃COOH, CH₃COO ⁻ , CH₃COOCH₃, CO₃²⁻ , SO₄²⁻ , PO₄³⁻
from period 3, octet expanded
Bond Energy
- Energy required to dissociate a bond in a gaseous state.
- Endothermic , (Bond Formation – Exothermic)
Bond Length
- Distance between nuclei where potential energy is minimized.
Polar vs. Nonpolar Bonds
- Bond : Electronegativity difference
- Molecular Polar – Asymmetric arrangement, Bond polarities not Cancel out, dipole Moments.
Resonance
- Not dynamic equilibrium
- Equivalent bond length and strength
- Only their average form exists (Bond order / # Resonance)
- Delocalized pi electrons.
- Enhance The Stability
- C₆H₆ , NO₃¹⁻ , CO₃²⁻ , SO₄²⁻ , PO₄³⁻ examples
Formal Charge
- Formal charge = original v.e – (# Lone e + # Bond )
- Zeros are preferred!
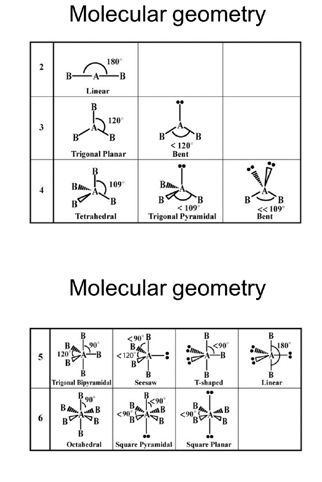
VSEPR Theory
- Valence Shell Electron Pair Repulsion.
- Minimizes repulsion:
- Bonding pair–bonding pair < bonding pair–lone pair < lone pair–lone pair.
- Molecular geometry vs Electron domain geometry
Hybridization
- Types: sp, sp², sp³.
Unit 3: IMF and Gas
Intermolecular Forces
- Strength – Intermolecular Forces determine melting and boiling points, Vapour pressure and Volatility. (Physical state)
London Dispersion Forces (LDF)
- Instantaneous dipoles, Temporary Foreces, Induced Dipoles.
- Present between all molecules, especially nonpolar.
- Increased by:
- Larger # electrons.
- Larger electron clouds.
- Greater polarizability.
Dipole-Dipole Interaction
- Present between polar molecules.
- Stronger than LDF but weaker than hydrogen bonding.
- Permanent Forces
Hydrogen Bonding
- Strongest intermolecular force.
- Examples: H bonded to F, O, or N.
Dipole-Induced Dipole Force
- Present between polar and nonpolar molecules.
- Stronger than LDF but weaker than dipole-dipole forces.
Ideal Gas Law and Partial Pressure
- Formula: PV = nRT
- Partial Pressure : Total pressure x mole Fraction
Kinetic Molecular Theory
- Ideal gas behavior
- Random & constant motion
- Perfectly elastic collision
- No intermolecular forces
- Negligible volume of particles (point-like)
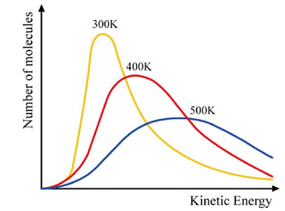
Kinetic Energy vs. Speed
- Although AVERAGE kinetic energy values are always constant for all gases
- Speed = v∝√(T/Mr)
Ideal Gas vs. Real Gas
- Ideal Gas
- Higher T, Lower P, Volum negligible, No Attraction
- Condition for less ideal:
- High P ➔ The combined volume of the particles becomes significant.
- Low T ➔ The intermolecular forces become significant due to their slow motion.
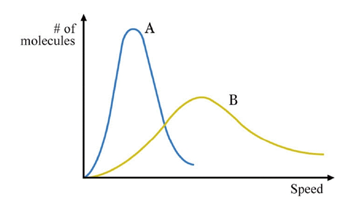
Maxwell-Boltzmann Distribution
Unit 4. Stoichiometry
Some Formulas
- Percent Yield: (actual yield / theoretical yield) × 100
- Percent Error: |theoretical − actual| / theoretical × 100
- Average Atomic Mass: Σ(mass of each isotope × % abundance)
Solubility Rule
- Every group 1A elements, NH₄⁺, NO₃⁻ containing salts are always soluble without exception.
- Strong Acid – H ⁺ , OH ⁻
Balancing Redox Reactions
- Balance element except O, H
- Balance O using H₂O
- Balance H using H⁺
- Balance charge using e⁻
Unit 5: Kinetics
Reaction Rate
- Definition: The slope of the graph of concentration vs. time.
- Change in concentration over time.
- Unit: M/s or mol/L·s.
Collision Theory
- Must Collide
- Proper Orientation
- Sufficient Energy greater than activation energy.→ Leads to effective collision (enables the reaction to occur).
Factors Affecting Reaction Rates
- Surface Area ( Smaller Size ) : Frequencies of Collision
- Concentration. – Frequencies of Collision
- Pressure and Volume. – Frequencies of Collision
- Temperature. 1) Velocity – Frequencies of Collision , More particles have Higher KE than Activation Energy
- Catalyst. – Lower Activation Energy → More particles have Higher Kinetic Energy than Activation Energy
Rate Law
- General Form: mA + nB → product
Rate = k × [A]ᵐ × [B]ⁿ - Reaction orders (m, n) are determined by experiment only.
- The unit of rate constant, k, depends on the overall reaction order.
- Rate constant is affected by temperature and the presence of a catalyst.
Rate Law Determination
Experimental rate law (concentration vs. rate).
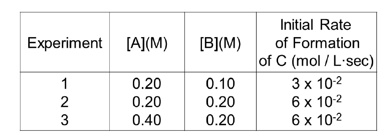
Rate law Diagram
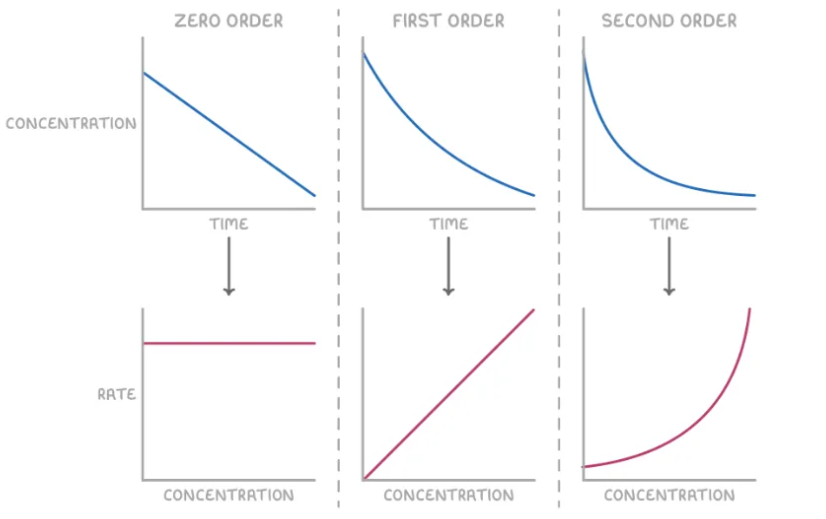
Integrated rate law (concentration vs. time).
- Zero Order: [A] = -kt + [A]₀
- First Order: ln[A] = -kt + ln[A]₀ (Half-life = 0.693/k).
- Second Order: 1/[A] = kt + 1/[A]₀
Reaction mechanism.
- A multistep reaction occurs as follows:
2A + B ⇌ C (fast, reversible) C + E → D + A (slow). - Overall Reaction Equation: A + B + E → D
Intermediate: **C** Catalyst: **A** - Rate Law:Rate = k[A]²[B].
Unit 6: Thermodynamics
Key Topics:
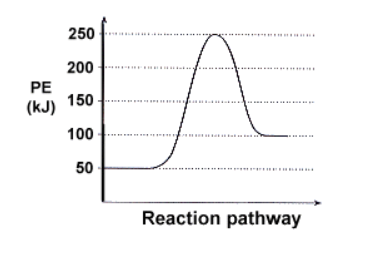
- Exothermic vs. Endothermic Reactions
- Standard Enthalpy Change (ΔH°) Calculations:
- Using calorimetric data: ΔH° = -q / mol , q = mct
- Hess’s Law: Sum of enthalpy changes of individual reactions
- Using bond energies, Combustaion energies: ΔH° = ΣB.E(reactants) – ΣB.E(products)
- Using formation energies
**ΔH° = Σproducts - ΣReactnats**
Entropy change (ΔS) Calculations
- Distribution higher → entropy change increases
- Calculation :
**ΔH° = Σproducts - ΣReactnats**
Spontaneity of Reactions
- ΔH°: Exothermic reactions (ΔH° < 0) are preferred.
- ΔS°: Higher entropy (ΔS° > 0) is preferred.
- ΔG°: Determines spontaneity (ΔG° = ΔH° – TΔS°).
Gibbs Free Energy (ΔG°):
- If ΔG° < 0: Reaction is thermodynamically favored. ΔG° = ΔH° – TΔS° ΔG° = -RT lnK ΔG° = -nFE°
- Temperature-dependent spontaneity:
- High T: ΔS-driven.
- Low T: ΔH-driven.
Unit 7: Equilibrium
- Meaning of Equilibrium
- Concentrations Constant
- Forward and reverse reaction rates are equal.
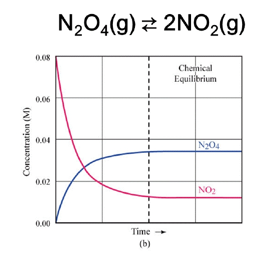
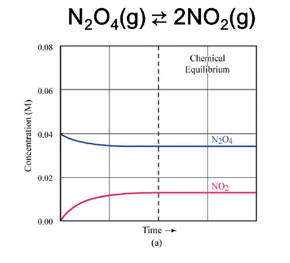
- Reversible vs. Irreversible Reactions
- Irreversible reactions (e.g., combustion, precipitation and gas produced)
- Expression:
Kₑq = [C]ᶜ[D]ᵈ / [A]ᵃ[B]ᵇ- Includes only gases and aqueous phases.
Factors Affecting Equilibrium
- Temperature:The only factor that changes Keq.
Relationship Between Keq and Q
- Q > Keq: Shifts left (toward reactants).
- Q < Keq: Shifts right (toward products).
- Q = Keq: The system is at equilibrium.
Le Chatelier’s Principle
- Types of Stress:
- Concentration Changes: Adjusts to restore equilibrium.
- Pressure Changes (via Volume):Affects gases; shifts to favor fewer or more moles of gas.
- Temperature Changes:Alters Keq (forward reaction is endothermic or exothermic reactions).
- Catalysts:Speed up the both reactions equally but do not affect Keq.
- Examples:
- N₂(g) + 3H₂(g) ⇌ 2NH₃(g) ΔH < 0
Manipulation of Equilibrium – rule
Ksp
- Definition of Ksp
- Soluble salt: AB₍aq₎ → A⁺₍aq₎ + B⁻₍aq₎ (Completely dissociated)
- Insoluble salt: AB₍ₛ₎ ⇌ A⁺₍aq₎ + B⁻₍aq₎ / Kₛₚ = [A⁺][B⁻]
- Basic Calculation
- If molar solubility = x:
- Kₛₚ of AB compound = x²
- Kₛₚ of AB₂ compound = 4x³
- If molar solubility = x:
- Common Ion Effect
Unit 8 : Acids and Bases
Common Acids and Bases
- Strong Acids: HCl, HBr, HI, H₂SO₄, HNO₃, HClO₄,
- Strong Bases: NaOH, KOH, Group 1A, 2A hydroxides.
- Weak Bases: NH₃, Fe(OH)₂, Al(OH)₃, Transition metal + OH
pH Calculation Formulas
- Strong Acids and Bases
pH (Strong Acid)=−log[H+] pOH (Strong Base)=−log[OH−]pH + pOH = 14 - Weak Acids pH = -log√(Ka × C)
pOH = -log√(Kb × C)
Percent Dissociation
%Dissociation=√(Ka/C) × 100
Acids and Bases with Water
- HCl + H₂O → H₃O⁺ + Cl⁻
- Cl⁻ + H₂O → HCl + OH⁻
- HF + H₂O ⇌ H₃O⁺ + F⁻
- F⁻ + H₂O ⇌ HF + OH⁻
Neutralization (Acid + Base , Carbonate and Metal)
- HCl + NaOH → H₂O + NaCl
- H⁺ + OH⁻ → H₂O
- HF + NaOH → H₂O + NaF
- HF + OH⁻ → H₂O + F⁻
- HCl + Na₂CO₃ → H₂O + CO₂ + 2NaCl
Ionic Equation: 2H⁺ + CO₃²⁻ → H₂O + CO₂ - HCl + Zn → ZnCl₂ + H₂
Ionic Equation: 2H⁺ + Zn → Zn²⁺ + H₂
Dissociation of a Polyprotic Acid
- H₂CO₃ + H₂O ⇌ H₃O⁺ + HCO₃⁻ Kₐ₁
- HCO₃⁻ + H₂O ⇌ H₃O⁺ + CO₃²⁻ Kₐ₂
Key Point:
- Kₐ₁ >> Kₐ₂
Neutralization of a Polyprotic Acid
- H₂CO₃ + 2NaOH → 2H₂O + Na₂CO₃
- Step 1: H₂CO₃ + NaOH → H₂O + NaHCO₃
- Step 2: NaHCO₃ + NaOH → H₂O + Na₂CO₃
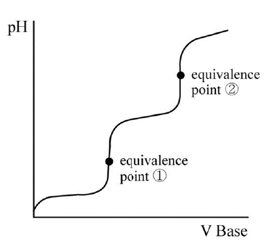
pH at the equivalence point
- Strong acid + strong base → neutral salt Example: HCl + NaOH → NaCl + H₂O / H⁺ + OH⁻ ⇌ H₂O
- Strong acid + weak base → acidic salt Example: HCl + NH₃ → NH₄Cl / H⁺ + NH₃ → NH₄⁺
- Weak acid + strong base → basic salt Example: HC₂H₃O₂ + NaOH → NaC₂H₃O₂ + H₂O HC₂H₃O₂ + OH⁻ → C₂H₃O₂⁻ + H₂O
- Weak acid + weak base → X
- CV = CV (Volume and Concentration)
- Whether acid is strong or weak, the volume of base is identical
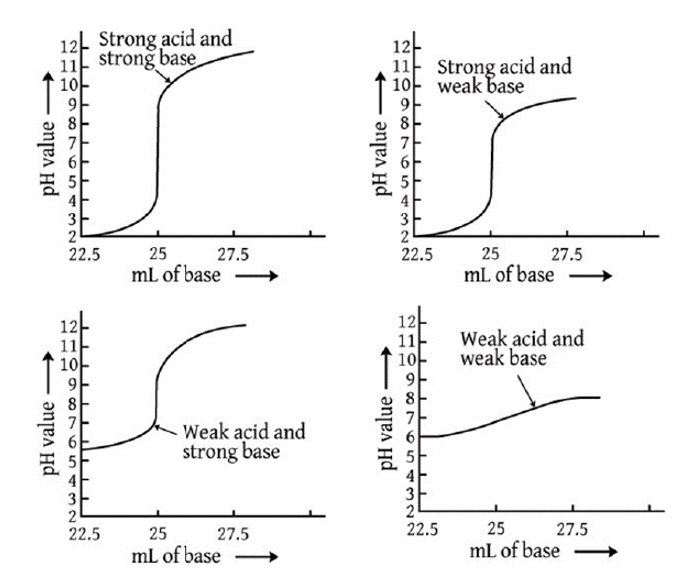
Hydrolysis of Salt
Why NH₄Cl is acidic
- Cl⁻ ion cannot react with water molecules, but NH₄⁺ does to a small extent.Example:
- NH₄⁺ + H₂O ⇌ NH₃ + H₃O⁺
Why NaCl is neutral
- Na⁺, Cl⁻ ions cannot react with water molecules.
Why NaC₂H₃O₂ is basic
- Na⁺ ion cannot react with water molecules, but C₂H₃O₂⁻ does to a small extent.Example:
- C₂H₃O₂⁻ + H₂O ⇌ HC₂H₃O₂ + OH⁻
How to prepare a buffer
Composition (by 1:1)
- Weak acid + conjugate base HF + NaF / HC₂H₃O₂+NaC₂H₃O₂
- Weak base + conjugate acid NH₃ + NH₄Cl
Composition (by 2:1)
- Weak acid + strong base HF + NaOH / HC₂H₃O₂ + NaOH
- Weak base + strong acid NH₃ + HCl
Half-equivalence point
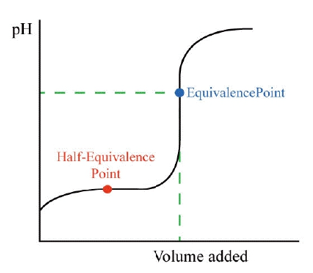
- Best buffer ratio!
- pH = pKa
Handerson-Hasselbalch equation
- pH = pKa + log [A⁻]/[HA]
- Used for only a buffered solution.
Choosing a good indicator
- pKa of the indicator needs to be close to the pH at the equivalence point.
- Lechatelier’s priciple
| Indicator | pKa | Usage |
|---|---|---|
| Methyl Red | 5.5 | S.A. + W.B. |
| Bromothymol Blue | 7.1 | S.A. + S.B. |
| Phenolphthalein | 8.7 | W.A. + S.B. |
W.A. and S.B. (buffering region)
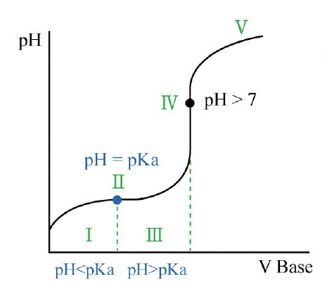
- pH < pKa: [HC₂H₃O₂] > [C₂H₃O₂⁻ ]
- pH = pKa:
[HC₂H₃O₂] = [C₂H₃O₂⁻ ] - pH > pKa:
[HC₂H₃O₂] < [C₂H₃O₂⁻ ] - higher Ka , higher Dissociation(Ionization)
Unit 9 : Cell and Others
Reaction:
- Cu² ⁺ + 2e⁻ → Cu , E° (Reduction Potential) =+0.34V
- Zn² ⁺ + 2e⁻ → Zn , E° (Reduction Potential) =−0.76V
Zn + Cu² ⁺ → Zn²⁺ + Cu, E° cell (Cell Potential) = 1.10 V
How Ecell changes with concentrations:
- Cell #1:
- [Cu²⁺] = 1.0 M, [Zn²⁺] = 1.0 M
- E° cell = 1.10 V
- Cell #2:
- [Cu²⁺] = 0.1 M, [Zn²⁺] = 1.0 M
- Ecell < 1.10 V
- Cell #3:
- [Cu²⁺] = 1.0 M, [Zn²⁺] = 0.1 M
- Ecell > 1.10 V
- Cell #4:
- [Cu²⁺] = 0.1 M, [Zn²⁺] = 0.1 M
- E° cell = 1.10 V, but it runs for a shorter time.
Equilibrium and Dead Battery
- Q = 1: E° cell = Ecell
- Q > 1:
- More Products (P), Less Reactants (R)
- Ecell < E° cell
- Closer to equilibrium
- Q < 1:
- Less Products (P), More Reactants (R)
- Ecell > E° cell
- Farther from equilibrium
Electrolysis
Electrolysis of molten salt:
- Reaction: 2NaCl → 2Na + Cl₂
- Na⁺ + e⁻ → Na (E° = -2.71 V)
- Cl₂ + 2e⁻ → 2Cl⁻ (E° = 1.36 V)
- Overall cell potential: E° cell = -4.07 V
- External electrical current > 4.07 V is needed.
- Electrolysis is non-spontaneous and requires energy.
Reactivity Comparison
- Order of reactivity for metals X, Y, Z:
- If X doesn’t react with Y(NO₃)₂ but reacts with Z(NO₃)₂:
- Reactivity: X < Z < Y (The highest Reducing agent)
- Tendency to lose electrons determines reactivity.
- If X doesn’t react with Y(NO₃)₂ but reacts with Z(NO₃)₂:
Metal Deposition
- Equation : q = It = nF
- How many grams of Al can be deposited from Al(NO₃)₃ solution by 2.0 A for 10 minutes?
- Answer: 0.11 g
- How long does it take to deposit 10.0 g of Cu from Cu(NO₃)₂ solution by 5.0 A?
- Answer: 6100 seconds or 1.0 x 10² minutes
- How many grams of Al can be deposited from Al(NO₃)₃ solution by 2.0 A for 10 minutes?
Laboratory
Solution Preparation (1): How to prepare 100 mL of 1.00 M NaCl solution
- Measure 5.85 g of NaCl using an electronic balance.
- Add the NaCl to a 100 mL volumetric flask.
- Add a small amount of water and swirl to dissolve completely.
- Fill the flask with water until the meniscus reaches the 100 mL mark.
Solution Preparation (2): How to prepare 500 mL of 0.10 M H₂SO₄ solution
- Wear safety goggles and gloves.
- Use M₁V₁ = M₂V₂ to calculate the volume of concentrated H₂SO₄ needed (25 mL).
- Add water to a volumetric flask first.
- Carefully add the acid to the flask.
- Add more water until the total volume reaches 500 mL.
Beer’s Law
- A = abc
- A: Absorbance (no unit)
- a: Absorptivity constant (M⁻¹cm⁻¹)
- b: Path length (cm)
- c: Concentration (M)
- Graph: Absorbance vs. Concentration
- Chromatography As solvent rises onto the paper, solute A~D reaches specific points depending on their intermolecular forces with the solvent molecules. In order of increasing interaction with the solvent: A < B < C < D
- Physical Separation Methods
- Filtration: Insoluble solid from liquid
- Distillation: Soluble solid from liquid or miscible liquids using boiling point differences
Lab Safety
- Never pour water to acid, as it might cause vigorous splattering due to rapid heat increase.
- Pour acid to water slowly to ensure proper absorption of heat.
- Never return residue of chemicals to the reagent container.
Titration
- Be careful with the buret:
- Rinse it with titrant twice before use. – water , titrant
- Choose an indicator with a pKa close to the equivalence point pH.
Gravimetric Analysis
- Example: A student determines the mass % of silver in a copper-silver alloy.
- Add saturated NaCl(aq) until no more precipitate forms.
- Filter, wash, dry, and weigh the precipitate.
- Repeat until a constant mass is achieved.
Dehydration of Hydrated Salt
- Cover the crucible to avoid mass loss.
- Measure repeatedly to ensure complete dehydration.
Gas Collecting via Water Displacement
- Adjust the graduated cylinder to equalize water levels.
- Consider vapor pressure, determined by temperature.
Significant Figures
- Examples:
- 0.010 g
- 100. mL
- log 0.010 = 2.00
- Rules:
- Addition/Subtraction: Use the fewest decimal places.
- Multiplication/Division: Use the fewest significant figures.